Rift Valley Fever
Rift Valley fever (RVFV) is a disease of viral origin, discovered in the early 1930s during an investigation into an outbreak of "enzootic hepatitis" (which affects a limited number of animals and is confined to certain herds) on a farm in Kenya's Rift Valley.
The epidemic caused numerous deaths among new-born lambs, along with increased mortality and spontaneous abortions among adult ewes, which reached into the thousands within just a few weeks. After noting that the event corresponded to a period of increased mosquito activity, the researchers hypothesised a possible link. The animals were then transported to the mountain highlands and protected with mosquito nets, which reduced the cases, further confirming the hypothesis.
The case was studied closely, both during and after the outbreak. However, another piece to the puzzle emerged when it was discovered that almost all of the shepherds had experienced fever and severe pain, suggesting that hundreds of human cases had also occurred.
Today, the disease still continues to cause the occasional outbreak, the largest of which occurred between 1978 and 1979 in Egypt, with some 200,000 human infections, 18,000 cases of sickness, and 600 deaths.
CAUSES
Rift Valley fever virus (RVFV) is a member of the Phlebovirus genus of the Phenuiviridae family. It is a anthropod-borne pathogen that affects ruminants, endemic to sub-Saharan Africa and the Arabian Peninsula.
RVFV contains a tripartite RNA genome with a small (S), medium (M) and large (L) genome segment.
RVF virions are spherical, consisting of an envelope and a ribonucleocapsid (RNP), measuring 80-120 nm in diameter. The viral envelope is covered with 122 capsomers, consisting of heterodimers of the Gn and Gc glycoproteins on an icosahedral lattice with T = 12 quasisymmetry.
TRANSMISSION
Numerous mosquito species are capable of transmitting RVFV, with Aedes being one of the principal vectors, while Culex, Anopheles and Mansonia play a minor but, in any case, important role in the development of outbreaks. Other arthropods, such as midges, ticks, and phlebotominae, can be infected with the virus and could potentially act as mechanical vectors.
A study of mosquitoes during a disease outbreak found that over 53 of the field-caught species tested positive for RVFV, while more than 65 species are described as potential vectors, the vast majority of which are theAedes and Culex mosquitoes.
Most human infections are due to contact with the blood or organs of an infected animal, which can occur at various stages of animal husbandry, from animal care to slaughter. Although not yet established, there is a possibility that the disease may also be transmitted by drinking raw milk. More rarely, although still possible, is transmission via mosquito bites and vertical transmission from mother to foetus, both of which have been documented.
For livestock, direct transfer between animals appears to be rare or non-existent, as evidenced by early experiments, which showed viraemic sheep are unable to transfer the virus to other animals. Recent research has shown that sheep with acute RVFV infections in close contact with immunocompromised lambs could not transmit the virus.
GEOGRAPHICAL DISTRIBUTION
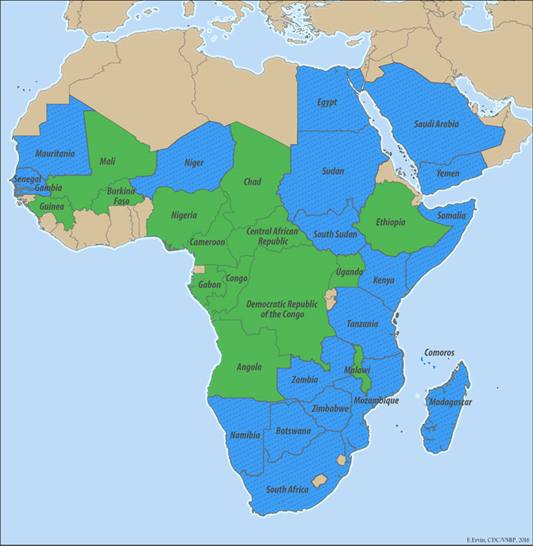
Rift Valley fever is confirmed in almost all of sub-Saharan Africa, Egypt, Saudi Arabia, and Yemen.
More specifically, the CDC in the United States, which monitors the status of the disease, reports that the region can be subdivided into three risk categories:
|
Countries reporting endemic diseases and substantial RVF outbreaks |
Egypt, Gambia, Kenya, Madagascar, Mauritania, Mozambique, Namibia, Saudi Arabia, Senegal, South Africa, South Sudan, Sudan, Tanzania, Yemen, Zambia, Zimbabwe |
|
Countries reporting very few cases, periodical virus isolation, or serologic evidence of RVF infection |
Angola, Botswana, Burkina Faso, Cameroon, Central African Republic, Chad, Democratic Republic of Congo, Ethiopia, Gabon, Guinea, Mali, Niger, Nigeria, Republic of Congo, Somalia, Uganda |
|
RVF status unknown |
All other countries |
SYMPTOMS
Human infections can lead to a broad spectrum of clinical outcomes. Although most cases fever that resolves on its own, it is estimated that 1-2% of infections result in a much more serious illness, often with a high level of mortality.
After an incubation period of 2-6 days, clinical symptoms of RVF include fever, headache, back pain, dizziness, anorexia, and photophobia (light-sensitivity). The fever can last several days, with a recovery period that ranges from a few days to a month. Some patients experience a two-stage febrile illness, in which there is a reduction in symptoms around the third day, with recurring fever 1-3 days later.
The severe form of RVF can cause a wide range of symptoms, such as hepatitis, jaundice, and haemorrhagic diseases. Patients with haemorrhagic fever have a very high mortality rate and usually succumb to the disease within one to two weeks after the onset of symptoms.
Other symptoms can include photophobia, reduced vision, blind spots, uveitis, retinitis, and retinal haemorrhage. The duration of the disease varies from chronic to resolution within a few weeks. It is estimated that ocular disease occurs in 2-5 % of mild RVFV infections, while the prevalence among people who present with severe disease can be over 10 %.
Serious and lasting problems can also occur shortly after the initial symptoms disappear, including reduced consciousness, hallucinations, confusion, dizziness, excessive salivation, weakness, paralysis, decerebrate posture, and hemiparesis.
DIAGNOSIS
There are several methods for diagnosing acute RVFV infection in cattle and humans, but all of them need to be performed in a laboratory.
One of these is the use of the ELISA technique to detect IgM antibodies typical of a recent infection. Highly sensitive and specific molecular RT-PCR methods can also be used to detect viral RNA. The virus can also be isolated in blood cultures sampled during the febrile stage (or post-mortem organ samples).
Because most human infections are asymptomatic or cause flu-like illness, the first signs of an RVF outbreak are near-simultaneous abortions in flocks of pregnant sheep, known as "abortion storms”.
TREATMENT
Most human cases of RVF do not require treatment. For patients with severe conditions, there is no specific treatment other than general supportive therapy, as there are no FDA-approved treatments for Rift Valley fever.
Symptoms, such as fever and muscle pain, can be treated with standard over-the-counter medications. Care for hospitalised patients is supportive, and also includes fluid replacement; medications that can affect liver and kidney function, or coagulation should be avoided.
PREVENTION
The risk of contracting Rift Valley Fever is considered rare for international travellers. More at-risk travellers are classified as people who choose rural locations, outside the usual tourist itineraries, as a travel destination.
There is no vaccine for RVF, so personal protection against mosquito bites is essential. Consequently, it is advisable to take the following precautions in endemic areas:
- Wear light-coloured clothes with long sleeves and long trousers that cover as much of the body as possible;
- Avoid wearing perfume;
- Apply insect repellent to exposed skin;
- Use pyrethroid-based insecticides that can also be sprayed directly onto clothing;
- Preferably stay in rooms with air-conditioning;
Contact with livestock, or wild animals, and their biological fluids should also be avoided in areas affected by outbreaks of Rift Valley Fever; If contact with animals is unavoidable, suitable personal protection equipment should be used.
Bibliografia:
- Daniel Wright, Jeroen Kortekaas, Thomas A. Bowden and George M. Warimwe. Rift Valley fever: biology and epidemiology. Wright et al., Journal of General Virology 2019;100:1187–1199 DOI 10.1099/jgv.0.001296;
- Karen L. Mansfielda, Ashley C. Banyarda, Lorraine McElhinneya, Nicholas Johnsona, Daniel L. Hortonb, Luis M. Hernández-Trianaa, Anthony R. Fooks. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. 0264-410,2015 Published by Elsevier Ltd.
- Tetsuro Ikegami. Molecular biology and genetic diversity of Rift Valley fever virus. Antiviral Res. 2012 September ; 95(3): 293–310. doi:10.1016/j.antiviral.2012.06.001.
- Amy Hartman. Rift Valley Fever. Clin Lab Med. 2017 June ; 37(2): 285–301. doi:10.1016/j.cll.2017.01.004.
- MaRyka R. Smith,1,3 Erin E. Schirtzinger,2,3 William C. Wilson,2 and A. Sally Davis. Rift Valley Fever Virus: Propagation, Quantification, and Storage. Current Protocols in Microbiology e92, Volume 55 Published in Wiley Online Library. doi: 10.1002/cpmc.92C 2019 John Wiley & Sons, Inc.





